Article Info
Year: 2025
Month: July
Issue: 3
Pages: 8-9
Human activity accelerates antimicrobial resistance, which occurs naturally in organisms. Drug resistance discreetly complicates infectious disease therapy and increases its spread, severity, and mortality. It makes medical interventions multiple times riskier. Multidrug-resistant organisms, mostly bacteria, emerged as a result [1]. Since 2017, the World Health Organization (WHO) has addressed medication resistance via a pathogen priority list. In 2019, the WHO named drug resistance one of the top 10 public health issues worldwide, responsible for 1.27 million deaths and warns that antibiotic resistance threatens global health and development. The median rates of 35–42% for certain bacteria across 76 countries hinder the effectiveness of common infection therapies [2].
The Kingdom of Saudi Arabia's (KSA) developing healthcare system makes it more sensitive to MDROs. Insufficient infection control and immigration from countries with poor healthcare practices contribute to an increase in MDRO infections. These include antibiotic overuse, prolonged hospital stays, and comorbidities like diabetes and chronic kidney disease.
To determine MDRO prevalence among King Saud Medical City (KSMC) hospitalized patients between May 2023 and October 2024 and investigate risk variables. The investigation will also characterize isolated MDROs' organisms and resistance patterns. We want to know how MDRO infections affect ICU admission and mortality. To determine MDRO infection distribution in KSMC departments and ICUs.
A retrospective cross-sectional study was conducted using electronic medical records of inpatients aged ≥18 years with culture-confirmed MDRO at King Saud Medical City between May 2023 and October 2024. Data included demographic characteristics, comorbidities, interventions, hospital stay details, and microbiological profiles. Descriptive statistics, chi-square tests, and binary logistic regression were employed.
Our analysis included 47,527 inpatients, of whom 1,785 had confirmed MDRO at KSMC, resulting in a 3.8% MDRO prevalence. Demographic data of patients displayed a mean age of 49.86 years with a standard deviation of 18.35, spanning from 18 to 100 years. The most significantly impacted age group was 30 to 39 years, at 19.4%. 70.5% of patients were male, and overall, 56.9% of patients were non-Saudi (NS).
In clinical outcomes, 47.7% underwent surgery, and the death rate was 20.2%. The average duration from sample admission to results was 26 days, with a standard deviation of 43.3, a median of 12 days, and a range of 1 to 425 days.
The predominant bacteria was carbapenem-resistant Klebsiella pneumoniae (CRKP), with a prevalence of 0.9%, succeeded by methicillin-resistant Staphylococcus aureus (MRSA) at 0.8% and subsequently carbapenem-resistant Acinetobacter baumannii (CRAB) at 0.5% (Figure 1). The allocation of patients within the facility was almost uniform between the Intensive Care Unit (ICU) and non-ICU, including 49.4% and 50.6%, respectively.
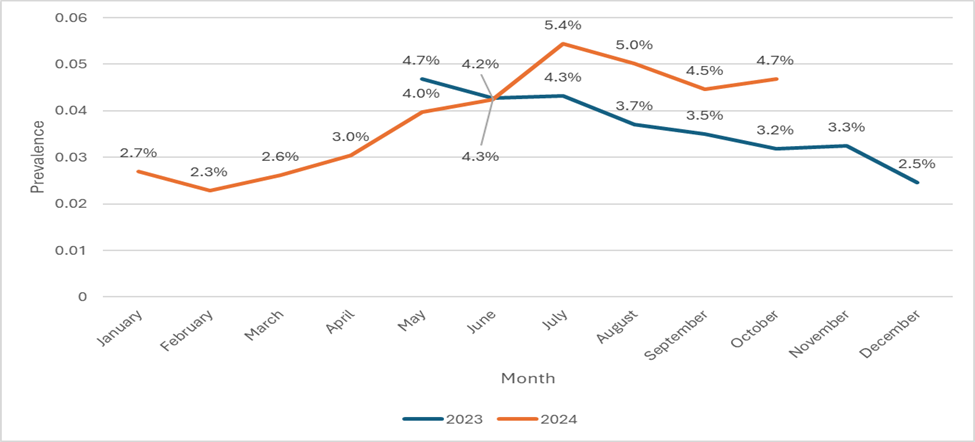
The temporal dispersion of collected samples increased to 1077 in 2024 from 708 in 2023. May had the most positive MDROs (4.7%) in 2023, followed by July and June (4.3%). In 2024, July had the most samples (5.4%), followed by August (5%) and October (4.7%) (Figure 2).
We identified various patient and clinical mortality risk variables in MDRO patients using a multivariate logistic regression model. Nationality showed lower mortality risks for Saudi patients compared to non-Saudi (OR = 0.5, 95% CI: 0.38–0.65, p < 0.001). ICU care environment significantly predicted mortality, with ICU patients having a threefold higher risk of death (OR = 2.98, 95% CI: 2.30–3.87, p < 0.001). Surgery patients had lower mortality rates (OR = 0.76, 95% CI: 0.59–0.97, p = 0.03). Compared to the reference age group (≤29 years), the 40-49 age group had significantly lower odds of death (OR = 0.35, 95% CI: 0.13–0.94, p = 0.04). However, this effect was not observed in other age groups, suggesting a nonlinear age-mortality relationship or unmeasured confounders. After adjustment, additional covariates did not affect mortality.
MDRO frequency was highest in 30-39 age group, implying that younger and middle-aged adults are more vulnerable to healthcare-related risk factors or are more mobile and utilize healthcare more frequently. In contrast to our data, research implies older age is a risk factor; groups over 70 years old had either lower admission rates or better infection control methods, which points to the importance of additional research [3,4].
Nationality affects MDRO slightly, as it is not a biological factor; however, research shows that nations may differ in healthcare practices, exposure, access to care, health-seeking behavior, or infection control. Further research is needed to identify if socioeconomic, behavioral, or healthcare factors are responsible [5].
In our data, CRKP, MRSA, CRAB, and Escherichia coli (ESBL) accounted for over 85% of the identified organisms at KSMC; this percentage is significantly higher than that reported in tertiary centers internationally, which suggests that there may be region-specific ecological burdens, which made it imperative to continue adjusting or adapting infection control measures based on the need. Activities should be examined for variations in practice among settings [6].
A crucial predictor is organism dispersion by care setting, which reveals significant heterogeneity in infection patterns. Staphylococcus aureus comprised 29% of ICU MDRO isolates. Similarly, in regional studies, this bacterium was detected in ICUs, indicating a significant clinical relationship with severely sick patients requiring invasive assistance. Klebsiella pneumoniae accounted for 30.9% of non-ICU MDRO isolates, suggesting that patients may carry it out asymptomatically. This study highlights the importance of comprehensive pathogenicity profiling to distinguish between colonization and invasive infection, as well as improved environmental cleaning methods in non-ICU settings, particularly with shared equipment.
Our findings advocate various practices for improving outcomes and limiting MDRO dissemination. First, infection prevention and control strategies should be reinforced in healthcare settings, particularly in the ICU, due to the increased risks in critically sick patients. Furthermore, tailored antimicrobial stewardship is essential for vulnerable groups, notably patients of specific ages with identifiable risk factors, to ensure optimal antibiotic use and address their increased infection risk. It would also be prudent to implement broader MDRO screening techniques, such as screening high-risk admissions or during peak seasons, to allow for early detection and isolation of carriers. Finally, we encourage further research into the behavioral and environmental drivers of MDRO transmission that underpin the observed temporal and seasonal trends, as well as the use of molecular epidemiology tools to better track the spread of these organisms and inform targeted interventions.
Figure 1: Prevalence of top 10 reported organism with drug resistance at King Saud Medical City between May 2023 and October 2024
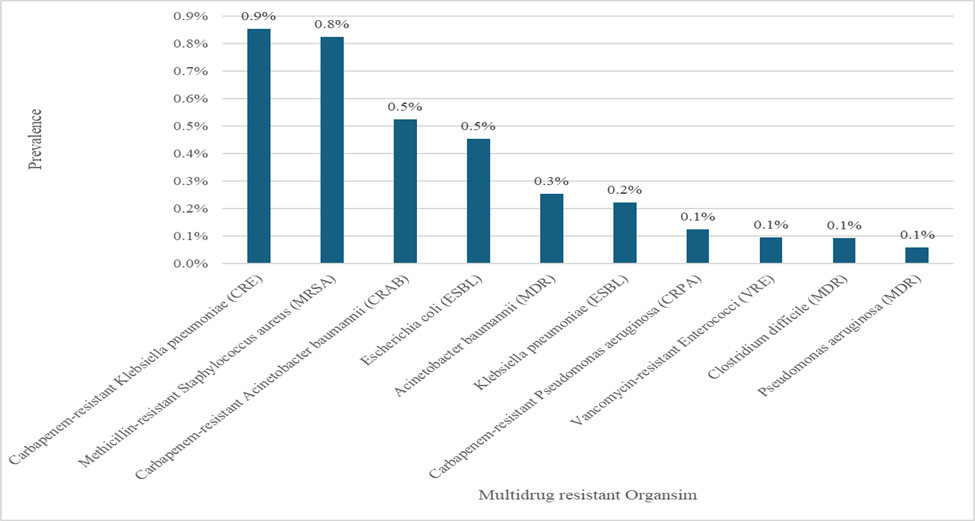
Figure 2: Multidrug-resistant organisms’ prevalence by Month (May 2023–Oct 2024) at King Saud Medical City
References:
1. Fullybright R. Characterization of Biological Resistance and Successful Drug Resistance Control in Medicine. Pathogens [Internet]. 2019 May 31;8(2):73. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6631572/
2. WHO. World Health Organization. 2023. Antimicrobial resistance. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistan…
3. Bhargava A, Riederer K, Sharma M, Fukushima EA, Johnson L, Saravolatz L. High rate of Multidrug-Resistant Organisms (MDROs) among COVID-19 patients presenting with bacteremia upon hospital admission. Am J Infect Control [Internet]. 2021 Nov [cited 2025 Jul 23];49(11):1441–2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8372431/
4. Rodríguez-Villodres Á, Martín-Gandul C, Peñalva G, Guisado-Gil AB, Crespo-Rivas JC, Pachón-Ibáñez ME, et al. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics [Internet]. 2021 Jun [cited 2025 Jul 28];10(6):680. Available from: https://www.mdpi.com/2079-6382/10/6/680
5. Zowawi HM. Antimicrobial resistance in Saudi Arabia: An urgent call for an immediate action. Saudi Med J [Internet]. 2016 Sep 1 [cited 2025 Jul 29];37(9):935–40. Available from: https://smj.org.sa/content/37/9/935
6. Yoo JH. Antimicrobial Resistance – The ‘Real’ Pandemic We Are Unaware Of, Yet Nearby. J Korean Med Sci [Internet]. 2025 May 13 [cited 2025 Aug 2];40(19). Available from: https://doi.org/10.3346/jkms.2025.40.e161
انتشار وعوامل الخطر وتوصيف الكائنات المقاومة لأدوية متعددة في مدينة الملك سعود الطبية 2023-2024
يسرع النشاط البشري من مقاومة مضادات الميكروبات، والتي تحدث بشكل طبيعي في دورة حياة الكائنات الحية. نتيجة لذلك ظهرت الكائنات الحية المقاومة للأدوية المتعددة، ومعظمها من البكتيريا. منذ عام 2017، تناولت منظمة الصحة العالمية مقاومة الأدوية من خلال قائمة أولويات مسببات الأمراض. في عام 2019، صنفت المنظمة مقاومة الأدوية كواحدة من أكبر 10 قضايا صحية عامة، وهي مسؤولة عن 1.27 مليون حالة وفاة، وتحذر من أن مقاومة المضادات الحيوية تهدد الصحة والتنمية العالميين. نمو نظام الرعاية الصحية في المملكة العربية السعودية يجعله حساساً للكائنات المقاومة لأدوية متعددة. ويساهم عدم كفاية مكافحة العدوى والهجرة من البلدان ذات الممارسات الصحية النقيصة في زيادة حالات العدوى.
أجريت دراسة تقصي مقطعي بأثر رجعي باستخدام السجلات الطبية الإلكترونية للمرضى المنومين في مدينة الملك سعود الطبية أكثر من 24 ساعة والذي تكون أعمارهم 18 عاماً وأكثر ولديهم عينات إيجابية لبكتيريا مقاومة لمضادات ميكروبات متعددة بين مايو 2023 وأكتوبر 2024. تضمنت المعطيات الخصائص للمرضى والبكتيريا المقاومة.
النتائج شملت 1785 عينة مريض إيجابية لبكتيريا مقاومة لمضادات ميكروبات متعددة من 47,527 مريضاً منوماً. بمعدل انتشار 3.8٪. بلغ متوسط الأعمار 49.86 سنة؛ 70.5٪ من الذكور، و56.9٪ من غير السعوديين، و50.6٪ في أماكن رعاية خارج وحدة العناية المركزة، 47.7٪ خضعوا لتدخل جراحي، نسبة الوفاة بين الحالات 20.2٪.
كانت أكثر أنواع البكتيريا انتشارًا هي الكلبسيلة الرئوية (0.9٪)، والمكورات العنقودية الذهبية (0.8٪)، والراكدة البومانية (0.5٪). وكانت مقاومة الكاربابينيم هي أكثر فئات المقاومة شيوعًا (43.2٪). وُجدت ارتباطات مهمة بين البكتيريا ومكان الرعاية. وقد حدد تحليل الانحدار فئات عمرية معينة، ورعاية وحدة العناية المركزة، والحالة الجراحية كمؤشرات للوفيات. شكلت أربعة أنواع من البكتيريا المقاومة لمضادات ميكروبات متعددة أكثر من 85٪ من الإصابات. هذه النسبة أعلى بكثير من تلك المبلغ عنها في المراكز على المستوى الدولي. بالإضافة الى وجود سيادة لبعض البكتيريا حسب منطقة العناية المركزة والغير مركزة.
ارتفع التشتت الزمني للعينات التي تم جمعها إلى 1077 في عام 2024 من 708 في عام 2023. كان لشهر مايو أكثر إيجابية (4.7٪) في عام 2023، يليه يوليو ويونيو (4.3٪). في عام 2024، كان لدى يوليو أكبر عدد من العينات (5.4٪)، يليه أغسطس (5٪) وأكتوبر (4.7٪).
تدعو النتائج توسعة الممارسات لتحسين والحد من نشر العدوى. أولا، ينبغي تعزيز استراتيجيات الوقاية من العدوى ومكافحتها في مرافق الرعاية الصحية، ولا سيما في وحدة العناية المركزة، بسبب زيادة المخاطر لدى المرضى المصابين بأمراض خطيرة. علاوة على ذلك، فإن الإشراف المخصص على مضادات الميكروبات أمر ضروري للفئات الضعيفة، ولا سيما المرضى من أعمار محددة مع عوامل خطر يمكن تحديدها، لضمان الاستخدام الأمثل. وسيكون من الحكمة أيضا تنفيذ تقنيات أوسع نطاقا لفحص الأدوية المتعددة الكثافة، مثل فحص حالات القبول عالية الخطورة أو خلال مواسم الذروة، للسماح بالكشف المبكر عن الناقلات وعزلها. أخيرا، نشجع على إجراء مزيد من البحث في الدوافع السلوكية والبيئية لانتقال العدوى التي تدعم الاتجاهات الزمنية والموسمية الملحوظة، بالإضافة إلى استخدام أدوات علم الأوبئة الجزيئية لتتبع انتشار هذه الكائنات بشكل أفضل وإبلاغ التدخلات المستهدفة.